- Home
- Auto Spent Catalyst Recycling
Auto Spent Catalyst Recycling
A “catalyst” is a substance that increases the speed of a chemical reaction without changing its own structure. These substances reduce the activation energy of the reaction, allowing it to happen faster. The effect a catalyst has on a reaction is called “catalysis.” Catalysis can be divided into two main types: “homogeneous catalysis” and “heterogeneous catalysis.” Although heterogeneous catalysis processes are still not fully understood, the most commonly used catalysts include high atomic number transition metals like platinum, osmium, rhodium, ruthenium, and palladium. In heterogeneous catalysis, catalysts are often broken down into smaller particles to increase surface area, resulting in better efficiency. Industrial catalysts are also widely used, especially in the petrochemical sector.
WORKING PRINCIPLES OF CATALYTIC CONVERTERS
Today, millions of vehicles are on the roads, each with the potential to pollute the air. In big cities, pollution from these vehicles can reach significant levels and cause serious problems. To reduce this issue, various laws and regulations are enforced to minimize pollution caused by exhaust gases. Car manufacturers have also made improvements to engines and fuel systems. One of the solutions to reduce air pollution is a device called the “catalytic converter,” which removes harmful components from exhaust gases before they are released into the air.
To control pollutants, devices are designed to keep the fuel-air ratio at an ideal level in engines. Theoretically, this ratio ensures that all oxygen in the air is used to burn all the fuel. For gasoline engines, the ideal fuel-air ratio is 14.7:1. However, this ratio can vary while driving, sometimes with a lean mixture (more air than fuel) or a rich mixture (more fuel than air).
The main emissions produced by a car engine include:
- Nitrogen Oxides (NOx)
- Carbon dioxide (CO2)
- Water vapor (H2O)
Most of these emissions (except for carbon dioxide) are harmless. However, the combustion process produces other harmful gases, which can damage the environment:
- Carbon Monoxide (CO) – A colorless, odorless, and toxic gas.
- Hydrocarbons or Volatile Organic Compounds (VOCs) – React with sunlight to create ozone (O3).
- Nitrogen oxides (NO and NO2, generally called NOx) – Cause smog and acid rain and can irritate the respiratory system.
Catalytic converters aim to minimize these three main pollutants: carbon monoxide, hydrocarbons, and nitrogen oxides.
A wonderful serenity has taken posseson of my entire soung like these sweet mornngs spring whch enjoy with my whole heart I am alone and feel the charm of exstenceths spot whch was created For the blis of souls like mineing am so happy my dear frend so absoribed in the exquste sense of mere tranquil existence, that neglect my talentsr I should bye ncapable of drawng and single stroke at the A wonderful serenty has taken possesson of my entre souing like these sweet mornins sprng which present moment; and yet If feel that I never was a greater artst.
ROLE OF CATALYTIC CONVERTERS IN REDUCING POLLUTION
Most modern vehicles have a “three-way catalytic converter.” The term “three-way” refers to controlling the three main pollutants: carbon monoxide, hydrocarbons, and nitrogen oxides. The catalytic converter uses two types of catalysts: a reduction catalyst and an oxidation catalyst. Both types are made by coating a ceramic structure with platinum, rhodium, and/or palladium. The main goal is to create a reaction in the exhaust gas with minimal use of catalyst material, maximizing the surface area for efficiency.
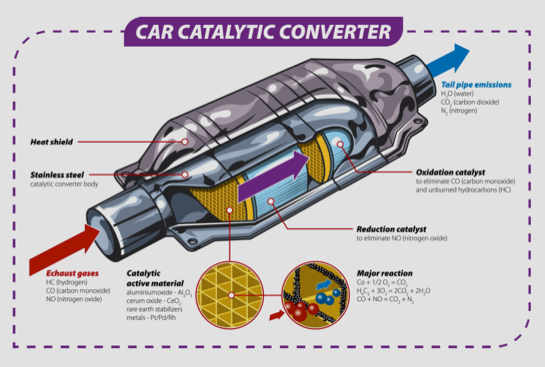
In a three-way catalytic converter, the following three reactions occur simultaneously:
Burning of carbon monoxide to carbon dioxide:
2CO + O2 → 2CO2
Reduction of nitrogen oxides to nitrogen:
NOx → O2 + N2
Conversion of unburned hydrocarbons (i.e. unburned fuel) to carbon dioxide and water, i.e. burning:
CxHy + nO2 → xCO2 + mH2O
FOR MORE INFORMATION ON AUTO SPENT CATALYST RECYCLING, YOU CAN CONTACT US.
Demcore Recycling

